BACKGROUND
Several studies have highlighted the poor outcomes observed among patients with relapsed/refractory multiple myeloma (MM), particularly those with triple-class refractory (TCR; refractory to at least one PI, one IMiD, and one anti-CD38 mAb) disease. The aim of this study was to describe the longitudinal changes in quality of life (QOL) and other patient-reported outcomes (PROs) among TCR MM patients in real-world clinical practice who initiate a new line-of-therapy.
METHODS
A prospective, multi-site observational study of patients with TCR MM was conducted through the Mayo Clinic system. Patients initiating a new post-TCR therapy (defined as the index date) were followed for a 12-month observation period during which PRO surveys were administered for the first 6 months. For patients initiating a CAR-T therapy, a survey assessment was conducted at apheresis, lymphodepletion chemotherapy, and every month after T-cell infusion until Month 6. All other patients received a survey assessment before index and then every month thereafter until Month 6. PRO measures included the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire (QLQ-C30; including the Global Health Score [GHS]), the EORTC Multiple Myeloma Questionnaire (QLQ-MY20; including the Disease Symptoms [DS] domain score), the EORTC QLQ for Chemotherapy-Induced Peripheral Neuropathy (CIPN20), the EuroQoL-5 Dimensions (EQ-5D), the Patient Global Impression of Severity (PGIS), and the Patient Global Impression of Change (PGIC). Patient surveys were supplemented with retrospective chart reviews conducted 3 months and 12 months following the index date to obtain clinical information. Descriptive data were reported at each timepoint separately for patients who received CAR-T and non-CAR-T index therapies. The study is ongoing and this abstract reflects all patient visit data as of April 2023.
RESULTS
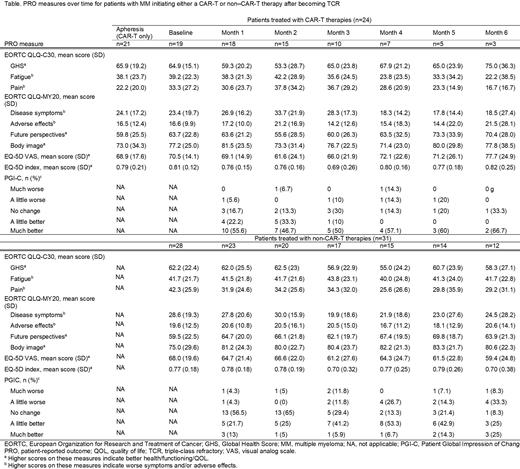
N=55 patients (60% male, 96% White, mean age 66 years) were included in this interim analysis. The median time interval between becoming TCR and the index date was 3.9 months (interquartile range [IQR]: 1.7, 15.5). Most patients had an ECOG score of 0 or 1 (53% and 38%, respectively) and were R-ISS I or II (36% and 54%, respectively); 53% of patients had high-risk cytogenetics and 20% had extramedullary disease. The median (IQR) number of prior treatment lines was 4 (4.0, 5.5). N=24 patients initiated a CAR-T therapy as their index therapy (equally split between ide-cel and cilta-cel); the remaining N=31 patients predominantly initiated a treatment regimen containing an IMiD (52%), a PI (55%), an anti-CD38 antibody (13%), or an alkylating agent (36%). Among CAR-T patients, QOL and disease-related symptoms worsened from baseline through Month 2-3 (eg, mean GHS was 65.9 at apheresis, 64.9 at lymphodepletion, and 53.3 at Month 2 [higher scores indicate better QOL]; mean DS domain score from the QLQ-MY20 was 24.1 at apheresis, 23.4 at lymphodepletion, and 33.7 at Month 2 [higher scores indicate more symptoms]; see Table 1). These decrements were followed by improvement back to baseline levels and, in some cases, improvement above baseline levels (eg, GHS was 67.9 and 65.0 at Months 4 and 5, respectively; DS was 18.3 and 17.8 at Months 4 and 5), though few patients had sufficient follow up to analyze QOL at later timepoints. Based on the PGIC, 77.8% of patients reported being “a little better” or “much better” as early as Month 1, though this number declined over time. Among non-CAR-T patients, QOL largely plateaued (GHS was 62.2 at baseline and 58.3 at Month 6), though disease symptoms improved over time (DS was 28.6 at baseline and 24.5 at Month 6; pain was 42.3 at baseline and 29.2 at Month 6). Based on the PGIC, 34.7% of patients reported being “a little better” or “much better” at Month 1, which improved to 50% by Month 6.
CONCLUSIONS
In this interim analysis of an ongoing prospective observational study, QOL and symptom measures generally worsened over the first few months for CAR-T patients in the real-world though improved to baseline levels and beyond at later time points. Patients did, however, directly report improvement in their disease state as early as Month 1 based on the PGIC. Non-CART patients generally showed a QOL plateau with some modest improvement in disease symptoms which was also reflected in their self-reported assessment of disease state improvement based on the PGIC.
OffLabel Disclosure:
Duh:Analysis Group, Inc.: Current Employment. Bobbili:Pfizer: Research Funding. Wang:Pfizer Inc.: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding. Mohan:Pfizer: Research Funding. Hlavacek:Pfizer Inc: Current Employment, Current equity holder in publicly-traded company. Schepart:Pfizer Inc: Current Employment, Current equity holder in publicly-traded company. Nador:Pfizer Inc: Current Employment, Current equity holder in publicly-traded company. DiBonaventura:Pfizer: Current Employment, Current equity holder in publicly-traded company.
Elranatamab is an investigational BCMA-CD3 bispecific antibody for the treatment of multiple myeloma.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal